Evolution of virulence, part I: definitions and basics
Evolution of virulence could be pretty important, both for
knowing about the evolution of emerging diseases that are coming
to infect us, and for biocontrol applications, and to answer
the basic ecological and evolutionary questions of why
Definition(s) of virulence
Virulence has had many definitions, depending on context and
tradition. Our ideal definition will be that virulence is the
decrease in the fitness of a standard host caused by association with
a parasite.
Fitness and components of fitness
The most common working definition of virulence is the mortality
caused by a parasite in a standard host. While mortality clearly
lessens fitness, it is not the only way that parasites can lower host
fitness (reduced fecundity in parasitized hosts is very common, with
the extreme being in cases of parasitic castration where fecundity may
be zero).
Indirect increases in mortality or decreases in fecundity (because
of changes in nutritional status or behavior), or interactions with
competition or predation, are even harder to measure but may be
important; we'll try to keep them in mind, even though we can
rarely quantify them. (This means that we need a standard
ecological context, as well as a standard host type, in order
to assay virulence.)
The standard definition of virulence in plant epidemiology is the
ability of a virus or fungus to infect a plant in the first place.
While low virulence in this sense (inability to infect the host)
leads to low virulence in our definition (the host's fitness won't
drop if it never gets infected in the first place), the
p.e. definition of virulence has more to do with host-specificity and
generality than with the issues we'll be discussing this week.
Virulence, resistance, and tolerance
We emphasized above that virulence is the harm done (reduction in
host fitness) to a standard
host in a standard ecological context.
Resistance, on the other hand, measures the harm (or
reduction in harm) done to a variety of hosts by a standard parasite.
The fitness of both host and parasite are a function of the
host-parasite relationship (and of the ecological context of that
relationship), but we try to break these apart. For example, if
parasite-induced mortality levels drop over evolutionary time, we'd
like to know whether that's caused by changes in the parasite
(virulence) or in the host (resistance). We wouldn't necessarily say
virulence had decreased.
Increases in host fitness via increased resistance usually imply
decreases in parasite fitness. However, another possibility (again
drawn from the plant epidemiology/pathology literature) is the
evolution of tolerance, where the host gains fitness without
necessarily reducing parasite fitness (e.g. by reduced levels of
immunopathology). This may be a step toward a commensal relationship,
where the former parasite can reproduce within the host but affect its
fitness negligibly.
Conventional wisdom
The conventional wisdom, repeated in many parasitology textbooks (and
expressed by many of you in your papers) is that a "well-adapted"
parasite harms its host little, if at all. The
evolutionary trajectory of a host-parasite association is towards a
mutualistic, or at least a commensal, symbiosis.
The less harm a parasite does, the better the host does, which means
(1) individual hosts are healthier (and survive longer); (2) host
populations are larger, providing more opportunities for parasite
offspring; (3) hosts may evolve weaker parasite defenses (although
this depends on the details of the costs and benefits of parasite
defense).
What is the evidence for this belief? Where did it come from?
- Many classical parasites appear to have relatively minor effects
on host fitness.
Parasitologists may not even include "harm to host" as part of the
definition of a parasite,
although pretty clearly if parasites didn't harm their
hosts at all we wouldn't study them so much.
(Parasites still get a whole lot more attention from
biologists than mutualists
and commensals do.)
There are a couple of potential fallacies in this
observation:
- evolutionary trajectories: as with body size and fecundity,
we're not asking how virulent parasites are but whether they become
more or less virulent over evolutionary time
- undetected costs: just because an animal appears functional
doesn't say how much better it would be doing if it were able to
get rid of its parasites, particularly if it could compete with
conspecifics that still had theirs
- Evolutionary trajectories:
- Syphilis (Treponema pallidum):
appeared in Europe, epidemic 1493-1510
(brought back from the New World? lots of debate).
(Primary symptoms: small skin lesions; secondary;
skin rash and fever; tertiary (ugh): cardiovascular and neurological
damage.) Appears to have become less virulent over
time (but? lots of complicating factors).
- Myxomatosis in rabbits: relatively benign in its original
associations (S. American rabbits) introduced as biocontrol in France,
England, most famously Australia. Careful tracking (with reference
strains of virus and rabbits) shows evidence for reduced virulence.
But there are very few really solid examples like
myxomatosis. Separating virulence from resistance can be hard.
- Effects of species jumping:
it seems that parasites are often more virulent
when they jump into a new species or population
("virgin soil" epidemics of measles, smallpox, etc.;
comparing SIV and HIV), or more virulent in "dead-end hosts", where
there is no selection for virulence (since the host always dies
without passing on parasite offspring).
However, this observation is also debated, since some have
argued that we only notice species jumps and dead-end infections when
they are virulent; if they die out harmlessly or quietly become
endemic in the population, we overlook them.
What's wrong (or overly simplified) with this picture?
Group selectionism (oh no!): why should parasites do anything for
the good of their hosts, or even for the good of their own species?
Short-term individual selection will often lead to higher virulence,
even if that means an eventual extinction of the host population and
the parasite lineage along with it. As we will see later there are
lots of special cases where lineage or kin selection may indeed be
operating in parasite evolution, but we have to be very careful using
it as a general explanation.
Tradeoff theory
A more recent theory of the evolution of virulence has taken a
different approach, arguing that evolutionary changes in virulence are
a result of tradeoffs between virulence and transmission of
disease between hosts.
This insight starts from looking at the fitness of a parasite in a
very simple epidemiological model, which gives an
expression for R0, the intrinsic reproductive
number of a disease or a parasite (the number of new infected
hosts generated in the lifetime of a parasite, in a completely
susceptible population). For a simple
microparasitic infection,
R0 = beta N / (m + v + r)
If all the parameters are independent, then it's easy to see what a
parasite should do: maximize transmission (beta) and minimize
virulence and recovery.
(This agrees with the "conventional wisdom", but from an individual
selection perspective: being less virulent just means that your own
host will survive longer, giving you longer to reproduce.)
However, theoreticians then pointed out that there is often a positive
relationship between virulence and transmission: the more resources
you take from the host to produce more offspring (more, larger, better
able to be transmitted etc.), the more likely you are to kill your
host or otherwise damage its fitness. There is no such thing as a
free lunch.
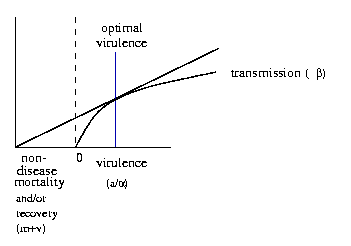
What is the optimal level of virulence?
Want the marginal value of increasing v
to be zero. If you're above that level, then you could
do better by reducing virulence and transmission slightly: the
extra time you have to reproduce more than offsets the reduction
in transmission. If you're below, then increasing transmission
improves your fitness (R0) more than the decrease caused
by reduced host lifespan.

The details of the shape of the transmission-virulence curve defines
how virulence of a particular host-parasite association will evolve.
Bad news: we don't often know the shape of the
transmission-virulence curve (although myxomatosis is a
counterexample).
Of course, an answer of "it depends" is probably a better
(although less satisfying) answer than "parasites always evolve
towards mutualism".
Also, as we'll see later there are qualitative differences
in modes of transmission etc. that we can draw qualitative,
testable conclusions from.
We can say some things directly by looking at the
following graphical model (to be explained in class):
Lenski & May: combining theories
As with Lively's attempt to combine Muller's ratchet
and the Red Queen, it's always interesting to try to synthesize
theories.
In particular, the tradeoff theory doesn't have group selection
problems, but it doesn't explain the observations (if we can believe
them) of reduced virulence in many host-parasite associations.
Lenski and May combine the two theories by combining ecology and
evolution. At any given population density, there is an optimal
(evolutionary stable) virulence level, which we can imagine
the population will evolve toward. However, virulent disease also
reduces the population density. Lower population densities mean
lower optimal transmission. There is a feedback between the ecology
and evolution of the system that drives virulence to a low (but not
zero) level.
(Picture)
Interesting theory but: when does it apply?
Parasite has to have ecological effects.
(e.g., may not work for syphilis and human diseases at least
in the modern era; might work for
myxomatosis and other diseases of natural populations).
Actual evidence of parasites reducing host population
levels is hard to come by, except in biocontrol situations.
Determinants of virulence
Disease-driven transmission
- Active transmission (rabies; sneezing and coughing).
- Predatory transmission (intermediate virulence? It doesn't help
to be lying on the bottom dead, but moving slowly might not be too
much of a problem for transmission).
- Necro-transmission: anthrax.
Vertical transmission
(cf castration, induced parthenogenesis)
Evolutionary interests of parasite and host
become (almost) identical; the same evolutionary
lineages transmit host genes and sexual disease.
There are all kinds of details that we won't go into
about the genetics of selfish gene transmission.
Sexually transmitted diseases
Sexual disease transmission often requires active participation by the
host, which doesn't work well if the host is debilitated by the
parasite
(although there are many ways that STDs are concealed from the
host: AIDS, syphilis both have long latent periods) ...
Poulin points out that
sexually transmitted diseases are also often quite host-specific
because reproductive isolation of hosts leads to isolation of
parasites.
Q: should this lead to lower virulence? Would it
require group or lineage selection?
"Passive" or vector-borne transmission
Hospitals, needles. Vectors that feed on debilitated
or dead hosts mean that there's less pressure for low
virulence in the hosts (although there's lots of pressure
for low virulence in the vectors).
Host-virus coevolution
The host is always trying to become more resistant or
tolerant (preserve as much of its fitness as possible),
while the parasite wants to stay at its "optimal" virulence;
if the host shifts damage down, then the parasite will try
to shift damage up to compensate.
This should lead to a discrepancy between the observed
virulence and the optimal virulence for the parasite, although
as yet we can't numerically predict the optimal virulence for
the parasite. However, in cases where we are able to suspend
either host or parasite evolution this theory of Ebert's predicts
qualitative shifts in virulence.
Evidence
Ewald's comparative data on disease severity in different
places, with different modes of transmission, etc.
Serial passage experiments
Vertical/horizontal transmission comparisons


