The Red Queen (and other explanations)

`Well, in OUR country,' said Alice, still panting a little,
`you'd generally get to somewhere else--if you ran very fast
for a long time, as we've been doing.'
`A slow sort of country!' said the Queen. `Now, HERE, you see,
it takes all the running YOU can do, to keep in the same place.
If you want to get somewhere else, you must run at least twice as
fast as that!'
Through the Looking Glass, Lewis Carroll
So what explains sex?
Muller's ratchet
Perhaps the simplest explanation, and one that we'll
have to test against, is Muller's ratchet.
The problem with asexuality is that, once
a deleterious mutation drifts to fixation, there's no way to get rid of
it. Every new mutation just adds to the mutational
load of a lineage, and there's no way to purge the
mutations from the population. Ooops.
This is yet another big topic we will just barely touch
on: Alex Kondrashov, James Crow, and many others have
worked for a long time at estimating the mutation rate,
and the distribution of effects of mutations, in
humans and other species and tried to estimate the
strength of Muller's ratchet. There is some evidence
that there is enough deleterious mutation to make sex
worth it.
Muller's ratchet only works in small populations
(in a large population deleterious mutations never
get fixed)
but there is also a deterministic theory of reduction of mutation load
in large populations, which depends on epistasis
(Kondrashov).
Hard (frequency-independent) habitat selection
Variation generated by sexual reproduction could allow populations
to inhabit niches that would not otherwise be accessible (none of
the variants in the clonal population can survive in them). This
works best if the open niches are transient - otherwise the
clonal populations will also be able to get into them eventually, once
appropriate mutations occur.
Tangled bank (soft/frequency-dependent selection)
Alternatively, the variation generated by sexual reproduction could
convey an advantage in competition/biotic interactions. The
distinction from the frequency-independent selection theory is that
we're now talking about fitness advantage relative to clonal
organisms, not absolute ability to survive in variable niches.
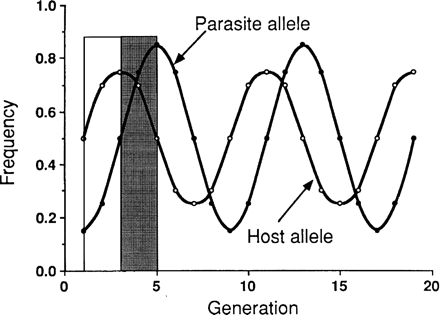
Red Queen
We get to the Red Queen by asking whether there is any process that
would generate a continuous need for variation. The (hypothesized)
antagonistic coevolution of hosts and parasites fits the bill; the
idea is that any common clone will get hammered by parasites that have
adapted to parasitize it. The best way to get around this is to
generate diversity via sexual reproduction.
There are lots of mathematical models with different genetic systems,
etc., etc..
The requirements of RQ:
- There must be heritable variation in host resistance
to parasites [otherwise hosts can't evolve].
- There is also heritable variation in the ability of parasites to
attack hosts (which is often called virulence in this
context, although we will define it differently later) [otherwise
parasite can't evolve].
- Parasite virulence and host resistance must be specialized
[otherwise either side would just get good at generalized attack
or defense]. There are several models of how this could work,
including gene-for-gene coevolution and
matching alleles (see below).
- Parasites cause (significant) harm to hosts
[otherwise they can't drive changes in host genetics].
Red Queen vs "arms race" dynamics
Note the difference between simple "arms races",
where both players (host and parasite) are simply
trying to maximize their overall performance
(resistance or virulence); whoever is stronger wins,
and the only limitation to the arms race is
the physical and resource constraint of
developing higher resistance or virulence.
In contrast, the Red Queen is about
recognition and evasion
mechanisms: either the host is trying to
recognize the host and the parasite is
trying to evade recognition, or the parasite
is trying to find chemical signals that match
the host properly and the host is trying
to avoid being matched. Red Queen dynamics
don't get anywhere, they just go 'round and
'round.
Gene-for-gene systems
|
pathogen phenotype |
| host phenotype |
avirulent (AA) |
avirulent (Aa) |
virulent (aa) |
| susceptible (rr) |
+ |
+ |
+ |
resistant (Rr) |
- |
- |
+ |
resistant (RR) |
- |
- |
+ |
A gene-for-gene interaction might start from susceptible hosts
(rr) and "avirulent" parasites (AA); the host cannot resist
the parasite. If the host gains the (dominant) resistant
allele it can resist avirulent parasites, but the (recessive)
virulence allele trumps the resistant allele and allows the
parasite to invade the host anyway.
The biochemistry of gene-for-gene systems is approximately
that the host needs to produce a protein/receptor that is
capable of detecting the parasite in order to trigger a
resistance response -- it can do this if it has at least
one resistance allele. However, the host receptor detects
a particular protein in the parasite phenotype, and if
the parasite can stop producing the protein, or produce
a modified version (i.e., it has no A alleles) it can
evade detection by the host.
Typically there are multiple gene-for-gene systems (multiple pairs of host
resistance alleles and parasite virulence alleles) within a given
host-parasite system. The host will try to detect the parasite
and resist it by trying out new resistance genes; the parasite
will try to evade the host by adding virulence genes that turn
off the detected molecule. Over time a Red Queen cycle can occur
because once a virulence allele has been driven to fixation,
the resistance allele is no longer useful and may be lost
by selection (if resistance carries a cost),
mutation or drift. Once this happens, the virulence gene is
no longer useful and it too may be lost by selection,
mutation or drift.
Matching-allele systems
|
pathogen phenotype |
| host phenotype |
AA |
Aa |
aa |
| rr |
- |
- |
+ |
Rr |
- |
+ |
- |
RR |
+ |
- |
- |
In matching alleles models,
the parasite "wins" if it matches the host's genotype.
One biochemical basis for this would be if the parasite
needed a particular protein that fitted the host in
some way, for example if it tries to evade
detection by mimicking the host's proteins.


